INTRODUCTION
About 50% of HCL patients (pts) relapse after chemotherapy with purine analogs (PA). After identifying the BRAF-V600E mutation as the genetic cause of HCL (Tiacci et al. NEJM 2011), we documented a high efficacy of the BRAF inhibitor vemurafenib in R/R pts (Tiacci, Park et al. NEJM 2015), especially when added to rituximab (Tiacci et al. NEJM 2021). HCL expresses bright CD20 and obinutuzumab (OBI) is a more effective anti-CD20 agent than rituximab (RTX) in other indolent B-cell neoplasms such as chronic lymphocytic leukemia and follicular lymphoma. RTX produces ~20% complete remissions (CR) in R/R HCL (Kreitman, ASH Educ Program 2012), while OBI activity in this setting is unknown.
METHODS
In the ongoing academic, phase-2, multi-center trial HCL-PG04, R/R HCL pts needing treatment due to cytopenia(s) received OBI 1000 mg intravenously on days 1-8-15 of cycle 1 and on day 1 of cycles 2-6 (1 cycle = 28 days). CR required resolution of cytopenias (platelets ≥ 100,000/mmc; neutrophils ≥ 1500/mmc; hemoglobin ≥ 11 g/dl), no palpable splenomegaly and no leukemic cells on morphologic analysis of the bone marrow biopsy, as assessed one month after the last OBI dose. Minimal residual disease (MRD) was analyzed by BRAF-V600E specific PCR in the bone marrow aspirate (sensitivity: 0.05% mutant alleles).
RESULTS
We enrolled 26 pts (M/F 24/2; median age 62 years, range 39-80) with a median of 2 prior therapies (range 1-7), including 7 refractory to PA (26%) and 2 to RTX (8%). Median values of disease burden parameters at baseline were: 70% BM HCL infiltration, 830 neutrophils/mmc, 62000 platelets/mmc, 14 g/dl hemoglobin, and 16 cm of longest spleen diameter (in 16/26 splenomegalic patients; 1 pts. had been splenectomized and 9 were not splenomegalic at baseline).
Toxicity was as expected, largely of grade (G) 1-2, always reversible and mainly consisting in: infusion reactions (G1-2 92%; G3 4%, in a single pt allergic to previous RTX who discontinued OBI); asymptomatic increase of liver enzymes (G1-2 19%; G3 GGT 15%; G3 ALT 4%); transient worsening of baseline G1-G3 thrombocytopenia to G3 (23%) or G4 (35%), with only 1 minor bleeding (4%, G1 epistaxis); transient neutropenia (G2 4%; G3 15%; G4 15%); and rare infections (G1-2 herpes labialis 8%; G3 pneumonia 4%).
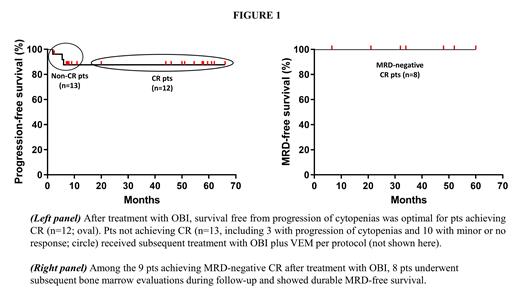
CR was reached in 12/25 evaluable pts (48%), including 2 pts with incomplete platelet recovery (~70000-90000/mmc) and 2 pts with delayed resolution of palpable splenomegaly. Notably, CR was achieved in 5/7 pts refractory to PA and 1/2 pts refractory to RTX. Furthermore, CR was negative for MRD in 9/12 pts (75%, including 3 pts with delayed MRD clearance), something never observed with vemurafenib. However, the kinetics of cytopenia resolution was slower compared to our previous vemurafenib trial, in that in pts obtaining CR with OBI neutrophil recovered to ≥1500/mmc after a median of 7 weeks (vs 4 weeks with vemurafenib) and platelets recovered to ≥100000/mmc after a median of 8 weeks (vs 2 weeks with vemurafenib). Prior exposure to RTX (n=12 pts) did not compromise OBI efficacy (5 CR, all MRD-negative, in 11 evaluable pts).
In pts achieving CR with OBI, progression-free and MRD-free survival were both 100% at 56 months (range 20-66) and 48 months (range 6.5-60) of median follow-up, respectively (Fig.1). The 13 non-CR pts (minor response 7; no response 3; progression 3) were effectively salvaged with another course of OBI combined with vemurafenib, which will be presented at the meeting.
CONCLUSIONS
OBI frequently produced deep and durable responses in R/R HCL, to an extent apparently greater than RTX, thus qualifying as an active and safe chemotherapy-free strategy in this setting.
OffLabel Disclosure:
Tiacci:Kite-Gilead: Consultancy; Deciphera: Consultancy; Innate Pharma: Consultancy. Zaja:Sobi: Honoraria, Research Funding; Grifols: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Pulsoni:MSD: Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria, Speakers Bureau; SANDOZ: Honoraria, Speakers Bureau; TAKED: Consultancy, Honoraria, Speakers Bureau; GILEAD: Consultancy, Honoraria, Speakers Bureau; Bristol Myers Squibb: Honoraria, Speakers Bureau; JANSSEN: Honoraria.
This presentation include information regarding obinutuzumab (anti-CD20 antibody) in the context of Hairy Cell leukemia.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal